Abstract
Background: Non-endemic Burkitt lymphoma (BL) is a rare B-cell malignancy characterized by extreme tumor proliferation, frequent extranodal involvement, and the genetic hallmark of a MYC gene rearrangement. Despite an often dramatic initial presentation featuring tumor compression of vital organs and/or spontaneous tumor lysis syndrome, most patients who survive intensive immunochemotherapy induction are cured. Despite limited evidence of benefit, the current NCCN guidelines recommend that BL patients in CR are reviewed every 3 months for 2 years and every 6 months thereafter.
Aims: To investigate outcomes, including relative survival and relapse risks conditional on event-free survival (EFS) milestones, in an international study of real-world BL patients treated with intensive immunochemotherapy.
Patients and methods: This is a retrospective study of newly diagnosed BL patients identified from relevant population or hospital-based registers in Australia (Perth), Denmark (the Danish Lymphoma Registry), Sweden (the Swedish Lymphoma Registry) and Norway (Health Region South East). Patients who met the following criteria were included irrespective of HIV status: 1) age ≥18 years at diagnosis, 2) diagnosed during the period 2005-2017, 3) histology and immunohistochemistry consistent with BL, 4) MYC translocation detected by fluorescence in situ hybridization (FISH), and 5) intensive first line immunochemotherapy including rituximab (R-CHOEP or more intensive). Patient data were collected from registers and chart reviews. Overall survival (OS) was defined as time until death and EFS was defined as time to death, relapse/progression, or unplanned treatment, whichever came first. Prognostic features at baseline were evaluated using univariate Cox models with EFS as outcome. Standardized mortality ratios (SMRs), conditional relative survival estimates, and relapse risks were computed for the subset of patients achieving complete remission (CR or CRu), with follow-up measured from response evaluation and from different EFS milestones.
Results: In total, 159 patients fulfilled the inclusion criteria of the study. The median age was 48 years (range 18-81) and the male:female ratio was 2.9. The baseline characteristics included stage III-IV (75%), elevated serum LDH (75%), extranodal involvement (83% - bone or bone marrow in 42% and CNS in 8%), B symptoms (60%), and ECOG performance score >1 (30%).
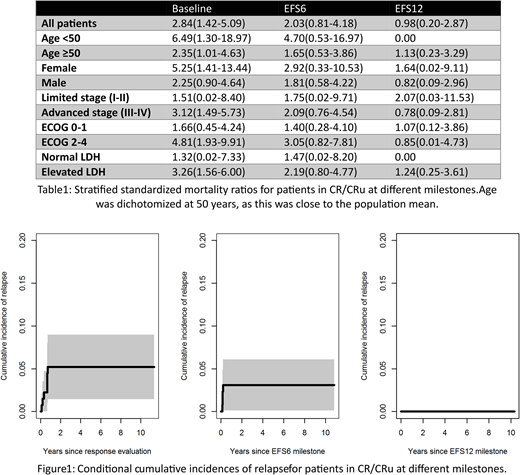
The chemotherapy protocols used were CHOEP (2%), DA-EPOCH (13%), HYPER-CVAD (26%), CODOX-M/IVAC (28%), BFM or GMALL (31%), and others (1%). Clinical tumor lysis syndrome defined by severe electrolyte derangement, renal impairment, and/or cardiac arrhythmia, was noted in 16% of the patients, but no fatal episodes occurred. The overall response rate to first-line treatment was 88% (87% in CR/CRu) with 67% assessed by PET technology. The five-year EFS and OS estimates for the total population were 75% (95% CI 68-82%) and 82% (95% CI 76-88%), respectively, and the EFS and OS of patients <50 years of age were 81% (95% CI 71-90%) and 89% (95% CI 82-96%), respectively. In univariate analyses, age, advanced stage, and elevated LDH were associated with worse EFS. Among patients in CR/CRu (n=137), the 5-year EFS and OS from the time of response evaluation were 86% (95% CI 79-93%) and 91% (95% CI 86-96%), respectively. The 5-year cumulative incidence of relapse from the time of response assessment was 5% (95% CI 1-9%) in CR/CRu patients. The corresponding estimate was 3% (95% CI 0-6%) for patients alive after 6 months without events (EFS6), whereas no patients relapsed after 12 (EFS12, Figure 1). For CR/CRu patients, the 5-year relative survival after EFS6 and EFS12 was 97% (95% CI 93-102%) and 100% (95% CI 97-103%), respectively. In most subgroups, the SMRs gradually decreased with increased remission duration (Table 1).
Conclusions: Outcomes of adult BL patients treated with intensive immunochemotherapy are excellent with no relapses occurring for patients reaching EFS12 and with a normalized relative survival after EFS6. For patients reaching EFS12, follow-up in late effects clinics, or discharging to primary care providers with a focus on survivorship issues rather than detection of recurrent lymphoma, should be considered. Updated analyses including patients from British Columbia (Canada) and the University of Iowa/Mayo Clinic SPORE MER register (USA) will be presented at the meeting.
Molin:Takeda Pharmaceuticals: Research Funding; Bristol-Myers Squibb: Honoraria; Roche Holding AG: Honoraria; Merck & Co., Inc: Honoraria. Ekstroem Smedby:Janssen Pharmaceuticals: Other: The Department have recieved partial funding from Janssen Pharmaceuticals.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal